Research Interests
- Accurate ab initio calculations on molecules including transition metals.
- Supramolecular systems: Fullerene crown ethers, complexes of porphyrins, encapsulated complexes.
- van der Waals systems.
- Organic reactions, reaction paths, catalysis.
- Solid state: Interactions and adsorption of small molecules on surfaces, magnetic nanostructures.
- Molecular logic gates, sensors, photoinduced charge transfer processes.
- Candidates for drugs. Photosensitizer candidates for photodynamic therapy.
- Theory
Recent Published Studies
- Encapsulated Complexes
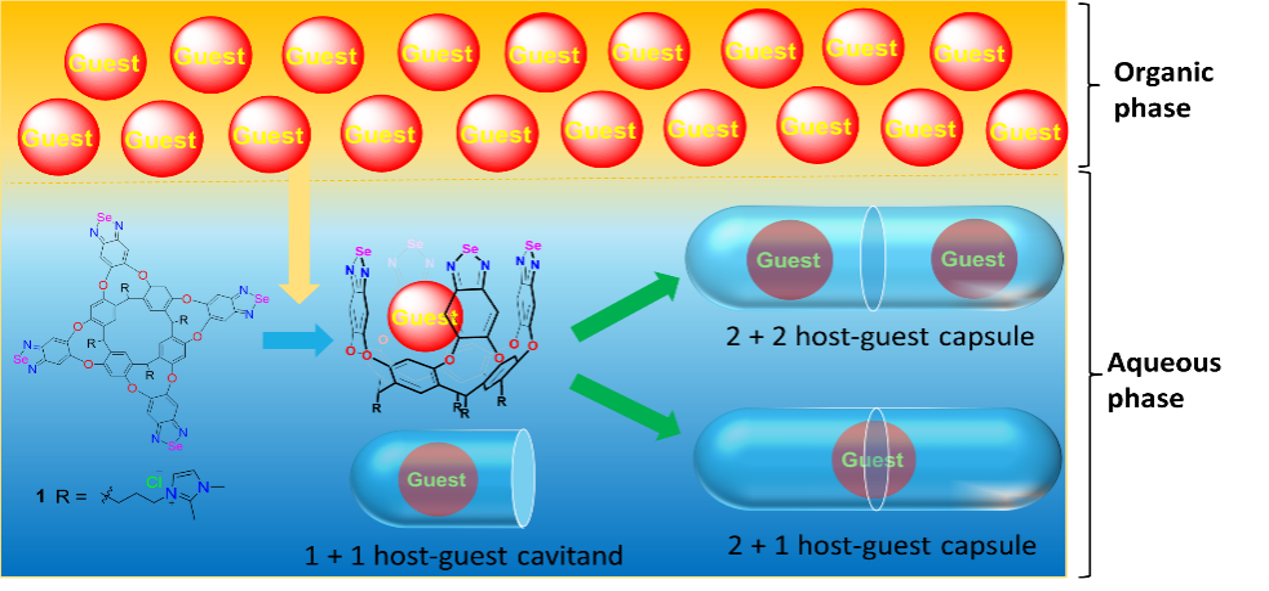
Supramolecular capsules present many diverse applications in sensing, transport, catalysis, material science and separation technologies. They are desirable containers for the study of molecular behavior in small spaces. The encapsulation of molecules in cages assembled by a variety of forces including hydrogen bonding, metal?ligand interactions, chalcogen bonding, and covalent bonding are studied. Recently, we found that cages from with chalcogen bonding present unusually high magnetic anisotropies in their interiors. Different factors contribute to the observed magnetic anisotropy of the capsule's interior: polarizability, inductive electric field effects. Additionally, chalcogen bonding persists well in water, in competition with the forces of hydrogen bonds.
Relevant publications
F.-Ur Rahman, D. Tzeli, I. Petsalakis, G. Theodorakopoulos, P. Ballester, J. Rebek, Jr., Y. Yu, "Chalcogen Bonding and Hydrophobic Effects Force Molecules into Small Spaces" J. Am. Chem. Soc. 142, 5876 (2020)
D. Tzeli, I. D. Petsalakis, G. Theodorakopoulos, F.-U. Rahman, P. Ballester, J. Rebek, Jr., Y. Yu, "Aromaticity and Chemical Bonding of Chalcogen-bonded capsules featuring enhanced magnetic anisotropy" ChemPhysChem 21, 2187 (2020).
D. Tzeli, I. D. Petsalakis, G. Theodorakopoulos, F.-U. Rahman, Y. Yu, J. Rebek, Jr., "The role of electric field, peripheral chains, and magnetic effects on significant 1H upfield shifts of the encapsulated molecules in chalcogen-bonded capsules", Phys. Chem. Chem. Phys. 23, 19647 (2021)
- Accurate ab initio calculations on molecules including transition metals.
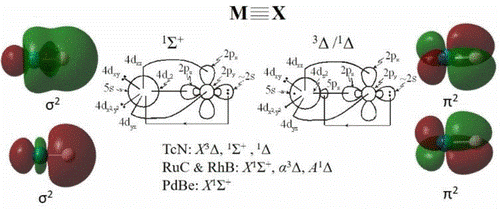
Nature of Chemical Bonding: Multiple bonds between atoms are one of the most fundamental aspects of Chemistry. Double and triple bonds are quite common, while quadruple bonds are a true oddity and very rare for the main group elements. Identifying molecules containing quadruple bonds is very important and even more so the determination of the necessary requirements for the existence of such bonds. There are two necessary requirements for the occurrence of quadruple bonds: i) the existence of low-lying atomic states that have low-lying unoccupied orbitals that can receive electrons via dative bonds and ii) atoms with double occupied orbitals that can form dative bonds.
Relevant publications
D. Tzeli and I. N. Karapetsas, "Quadruple Bonding in the Ground and Low-Lying Excited States of the Diatomic Molecules TcN, RuC, RhB, and PdBe" J. Phys. Chem. A 124, 6667 (2020)
D. Tzeli, "Quadruple chemical bonding in the diatomic anions TcN-, RuC-, RhB-, and PdBe-" J. Comput. Chem. 42, 6667 (2021).
T. Depastas, A. Androutsopoulos, D. Tzeli, "Analysis of chemical bonding of the ground and low-lying states of Mo2 and of Mo2Clx complexes, x=2-10". J. Chem. Phys. 157, 054302 (2022)
- Molecular Logic Gates
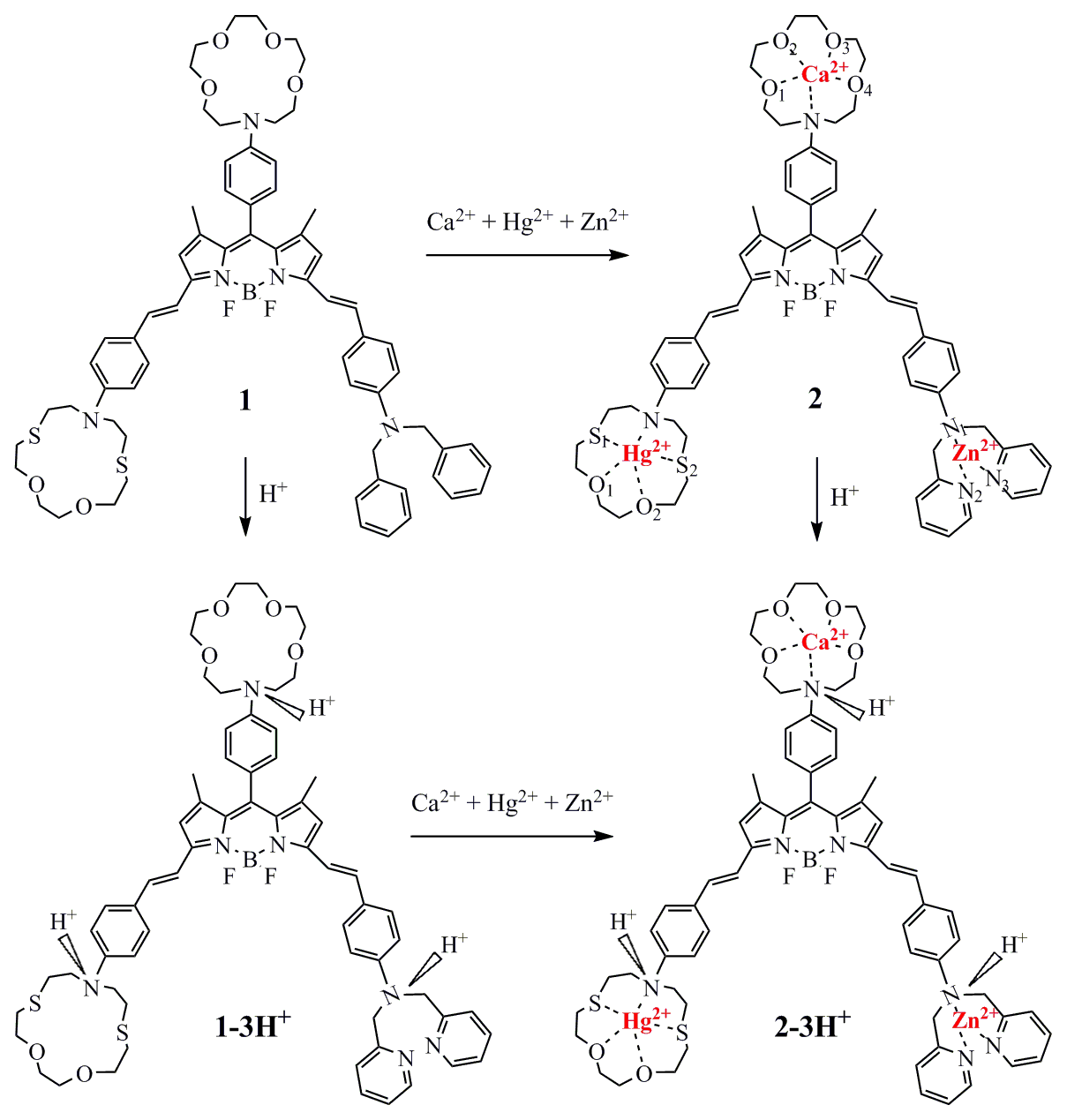
Molecules respond to changes in their environment, such as changes of pH, temperature, solvent, viscosity, the presence of various ions, of neutral species, and so on. As a result, in response to these modulations, molecules can exhibit differences in their ground or excited electronic states, with accompanying modifications in the absorption and/or emission intensity and wavelength. Such changes can be related to the operation of fluorescence chemosensors, molecular switches, and logic gates (via the familiar Boolean logic where the modulators correspond to the inputs and the observed changes correspond to the outputs). As a consequence, the field of molecular logic gates and generally of chemosensors and molecular switches is very active and many research groups around the world work on these subjects. Recently we studied molecule 1 which can be used as a 3 input AND molecular logic gate but only in an appropriate solvent. The selection of the solvent conditions is crucial for a species to act as an MLG candidate.

Relevant publications
D. Tzeli, I. Petsalakis, G. Theodorakopoulos "Theoretical study of the photophysical processes of a styryl-bodipy derivative eliciting an AND molecular logic gate response" Int. J. Quantum Chem. 119, e25958 (2019)
D. Tzeli, I. D. Petsalakis, G. Theodorakopoulos, "The solvent effect on a styryl-bodipy derivative functioning as an AND molecular logic gate" Int. J. Quantum Chem. 120, e26181 (2020).
C. E. Tzeliou, D. Tzeli, "3-input AND molecular logic gate with enhanced fluorescence output: The key atom for the accurate prediction of the spectra" J. Chem. Inf. Model. 62, 6436 (2022).
- Medicinal Chemistry
Computational study of compounds presenting anti-inflammatory and/or antioxidant activity via quantum mechanics (QM), multiscaling quantum mechanics/molecular mechanics (QM/MM) and molecular dynamics (MD) methods.
Study of 2,2'-dihydroxybenzophenones and derivatives. Significant anti-inflammatory activity of the compounds has been found by in vivo tests with their oxime and hydrazone derivatives showing that some derivatives compete with marketed drugs.
Relevant publications
D. Tzeli, P. Kozielewicz, M. Zervou, C. Potamitis, K. Kokkotou, B. Rak , A. Petrou, E. Tsolaki, A. Gavalas, A. Geronikaki, I. D. Petsalakis, P. G. Tsoungas, "2,2'-Dihydroxybenzophenones and Derivatives. Efficient Synthesis and Structure Endoscopy by DFT and NMR. Credentials as Potent Antiinflammatory Agents", Chem Select 1, 2426 (2016)
V. Vakali, M. Papadourakis, N. Georgiou, N. Zoupanou, D. Diamantis, U. Javornik, P. Papakyriakopoulou, J. Plavec, G. Valsami, A. Tzakos, D. Tzeli, Z. Cournia, T. Mauromoustakos, "Comparative interaction studies of quercetin with 2-hydroxyl-propyl-cycodextrin and 2,6-methylated-cyclodextrin", Molecules 27, 5490 (2022).
C. E. Tzeliou, M. A. Mermigki, D. Tzeli, "Review on the QM/MM methodologies and Their Application to Metalloproteins", Molecules 27, 2660 (2022).